Quality &
Compliance
We are a specialist manufacturer of defibrillator and ECG electrodes known for our design, validation and manufacturing excellence
Intelesens operates a Quality Management System which has successfully been certified to ISO 13485:2016 for medical devices.
Intelesens is dedicated to maintaining the highest quality standards in all that we do.

Highest
Quality
The highest quality of goods and services are provided to our customers

Customer
Requirements
Customer’s requirements are considered at all stages of the development and manufacturing process

Fit For
Purpose
All products manufactured are fully in accordance with the product’s intended use

High Standards
Throughout
Technical standards and regulations are maintained throughout each of each project
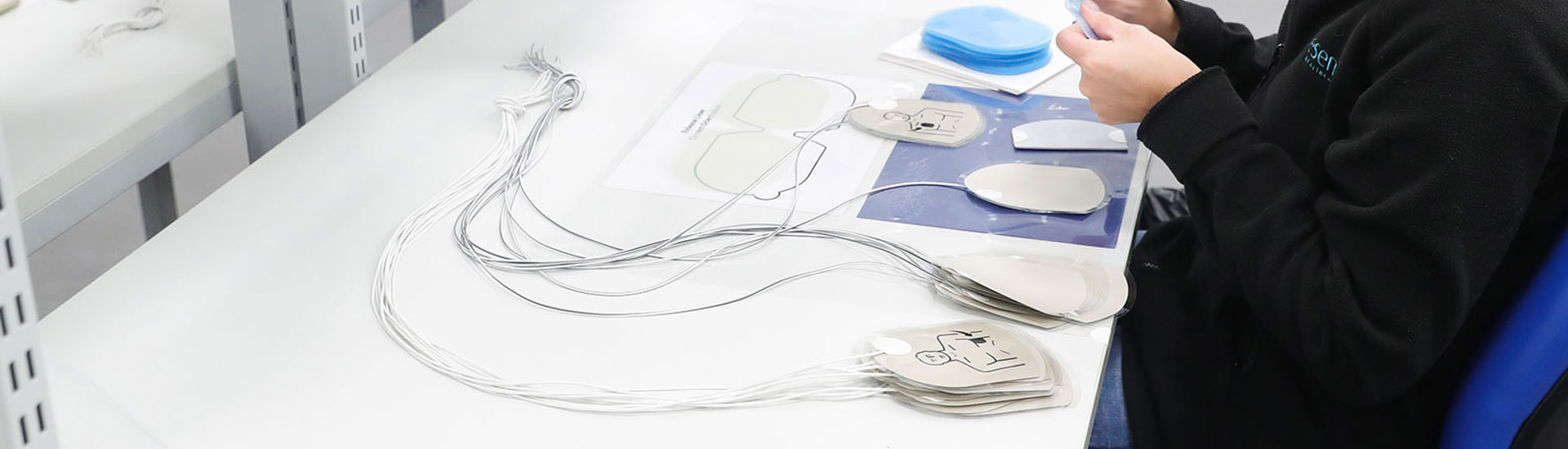
Quality Management is an active and engaging process
At Intelesens, we conduct periodic reviews of our quality management system and monitor the implementation and effectiveness of quality plans. We implement and
maintain a risk management approach in the processes of the Quality System and
in the life cycle of products. Our pursuit of our quality standards and operational
procedures are clearly defined in the Quality Manual, and strictly enforced throughout the company and the production of the full range of Intelesens products.
These procedures are in accordance with the requirements of ISO 13485 and are the minimum standard adopted by Intelesens. All Quality System procedures are mandatory and unauthorised deviations from these standards are not allowed. Procedures other than those defined, or otherwise required by the customer, may apply only after their effectiveness has been demonstrated and agreed by management.
You can download our ISO 13485:2016 for medical devices below

